| |||||
|
| |||||
INTRODUCTION AND OVERVIEW |
|||||
|
X-radiation
is created by taking energy from electrons and converting it into
photons with appropriate energies. This energy conversion takes place
within the x-ray tube. The
quantity (exposure) and quality (spectrum) of the x-radiation produced can be controlled by adjusting the electrical quantities (KV,
MA) and exposure time, S, applied to the tube. In
this chapter we first become familiar with the design and construction
of x-ray tubes, then look at the x-ray production process, and conclude
by reviewing the quantitative aspects of x-ray production.
| |||||
Function |
|
|
An x-ray tube is an energy converter. It receives electrical energy and
converts it into two other forms: x-radiation and heat. The heat is an
undesirable byproduct. X-ray tubes are designed and constructed to
maximize x-ray production and to dissipate heat as rapidly as possible.
The x-ray tube is a relatively simple electrical device typically containing two principle elements: a cathode and an anode. As the electrical current flows through the tube from cathode to anode, the electrons undergo an energy loss, which results in the generation of x-radiation. A cross-sectional view of a typical x-ray tube is shown in below. 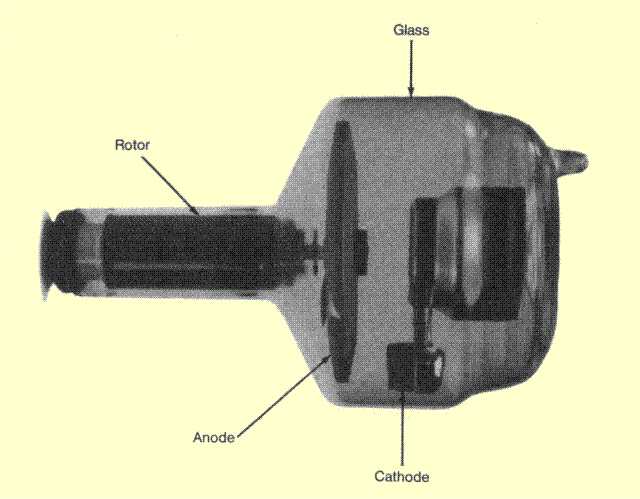 Cross-Section of a Typical X-Ray Tube | |
Anode |
|
|
The
anode is the component in which the x-radiation is produced. It is a
relatively large piece of metal that connects to the positive side of
the electrical circuit.
The anode has two primary functions: (1) to convert electronic energy
into x-radiation, and (2) to dissipate the heat created in the process.
The material for the anode is selected to enhance these functions.
The ideal situation would be if most of the electrons created x-ray
photons rather than heat. The fraction of the total electronic energy
that is converted into x-radiation (efficiency) depends on two factors:
the atomic number (Z) of the anode material and the energy of the
electrons. Most x-ray tubes use tungsten, which has an atomic number of
74, as the anode material. In addition to a high atomic number, tungsten
has several other characteristics that make it suited for this purpose.
Tungsten is almost unique in its ability to maintain its strength at
high temperatures, and it has a high melting point and a relatively low
rate of evaporation. For many years, pure tungsten was used as the anode
material. In recent years an alloy of tungsten and rhenium has been
used as the target material but only for the surface of some anodes. The
anode body under the tungsten-rhenium surface on many tubes is
manufactured from a material that is relatively light and has good heat
storage capability. Two such materials are molybdenum and graphite. The
use of molybdenum as an anode base material should not be confused with
its use as an anode surface material. Most x-ray tubes used for
mammography have molybdenum-surface anodes. This material has an
intermediate atomic number (Z = 42), which produces characteristic x-ray
photons with energies well suited to this particular application. Some
mammography tubes also have a second anode made of rhodium, which has an
atomic number of 45. This produces a higher energy and more penetrating
radiation, which can be used to image dense breast.
The use of a rhenium-tungsten alloy improves the long-term radiation
output of tubes. With x-ray tubes with pure tungsten anodes, radiation
output is reduced with usage because of thermal damage to the surface
| |
|
| |
Design |
|
|
Most anodes are shaped as beveled disks and attached
to the shaft of an electric motor that rotates them at relatively high
speeds during the x-ray production process. The purpose of anode rotation
is to dissipate heat and is considered in detail in another chapter.
| |
|
| |
Focal Spot |
|
|
Not all of the anode is involved in x-ray production. The radiation is
produced in a very small area on the surface of the anode known as the
focal spot. The dimensions of the focal spot are determined
by the dimensions of the electron beam arriving from the cathode. In
most x-ray tubes, the focal spot is
approximately rectangular. The dimensions of focal spots usually
range from 0.1 mm to 2 mm. X-ray tubes are designed to have specific
focal spot sizes; small focal spots
produce
less blurring and better visibility of detail, and large focal spots have a greater heat-dissipating capacity.
Focal spot size is one factor that must be considered
when selecting an x-ray tube for a specific application. Tubes
with small
focal spots are used when high image visibility of detail is
essential and the amount of radiation needed is relatively low
because of small and thin body regions as in mammography.
Most x-ray tubes have two focal spot sizes (small and large), which can be selected by the operator according to the imaging procedure. | |
|
| |
Cathode |
|
|
The basic function of the cathode is to expel the electrons from the
electrical circuit and focus them into a well-defined beam aimed at the
anode. The typical cathode consists of a small coil of wire (a filament)
recessed within a cup-shaped region, as shown
below.

Energy Exchange within an X-Ray Tube Electrons that flow through electrical circuits cannot generally escape from the conductor material and move into free space. They can, however, if they are given sufficient energy. In a process known as thermionic emission, thermal energy (or heat) is used to expel the electrons from the cathode. The filament of the cathode is heated in the same way as a light bulb filament by passing a current through it. This heating current is not the same as the current flowing through the x-ray tube (the MA) that produces the x-radiation. During tube operation, the cathode is heated to a glowing temperature, and the heat energy expels some of the electrons from the cathode. | |
|
| |
Envelope |
|
|
The anode and cathode are contained in an airtight enclosure, or
envelope. The envelope and its contents are often referred to as the
tube insert, which is the part of the tube that has a
limited lifetime and can be replaced within the housing. The majority of
x-ray tubes have glass envelopes, although tubes for some applications
have metal and ceramic envelopes.
The primary functions of the envelope are to provide support and electrical insulation for the anode and cathode assemblies and to maintain a vacuum in the tube. The presence of gases in the x-ray tube would allow electricity to flow through the tube freely, rather than only in the electron beam. This would interfere with x-ray production and possibly damage the circuit. | |
Housing |
|
|
The x-ray tube housing provides several functions in addition to
enclosing and supporting the other components. It
functions as a shield and absorbs radiation, except for the
radiation that passes through the window as the useful x-ray beam. Its
relatively large exterior surface dissipates most of the heat created
within the tube. The space between the housing and insert is filled with
oil, which provides electrical insulation and transfers heat from the
insert to the housing surface.
| |
THE X-RAY CIRCUIT |
|
|
The energy
used by the x-ray tube to produce x-radiation is supplied by an
electrical circuit as illustrated
below. The circuit connects the tube to the source of electrical energy, that in the x-ray room is often referred to as the generator. As described in another chapter, the generator receives the electrical energy from the electrical power system and converts it into the appropriate form (DC, direct current) to apply to the x-ray tube. The generator also provides the ability to adjust certain electrical quantities that control the x-ray production process.
The three principle electrical quantities
that can be adjusted are the:
The circuit is actually a circulatory
system for electrons. They pickup energy as the pass through the
generator and transfer their energy to the x-ray tube anode as described
above.
| |
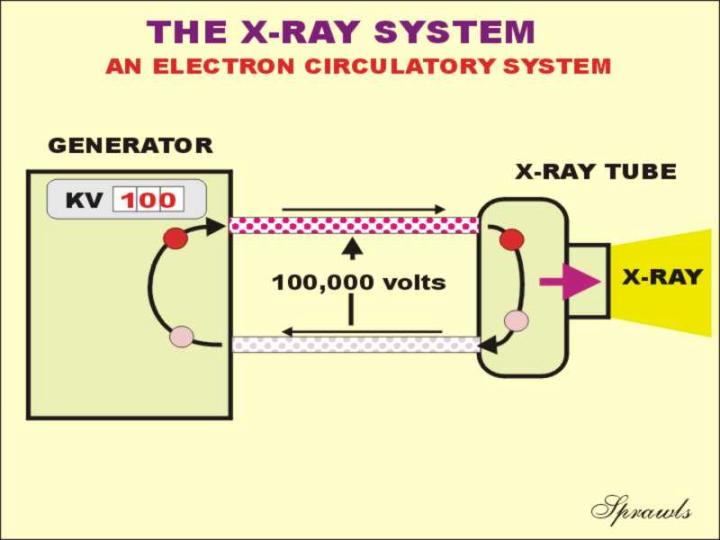 | |
ELECTRON ENERGY |
|
|
The energy that will be converted into x-radiation (and heat) is
carried to the x-ray tube by a current of flowing electrons
as shown above. As the electrons pass through the x-ray tube, they
undergo two energy conversions, as illustrated
previously: The electrical potential energy is converted into
kinetic
(motion) energy that is, in turn, converted into x-radiation and
heat.
| |
Potential |
|
|
When the electrons arrive at the x-ray tube, they carry electrical
potential energy. The amount of energy carried by each electron is
determined by the voltage or KV, between the anode and cathode. For each
kV of voltage, each electron has 1 keV of energy. By adjusting the KV,
the x-ray machine operator actually assigns a specific amount of energy
to each electron.
| |
Kinetic |
|
|
After the electrons are emitted from the cathode, they come under the
influence of an electrical force pulling them toward the anode. This
force accelerates them, causing an increase in velocity and kinetic
energy. This increase in kinetic energy continues as the electrons
travel from the cathode to the anode. As the electron moves from cathode
to anode, however, its electrical potential energy decreases as it is
converted into kinetic energy all along the way. Just as the electron
arrives at the surface of the anode its potential energy is lost, and
all its energy is kinetic. At this point the electron is traveling with a
relatively high velocity determined by its actual energy content. A
100-keV electron reaches the anode surface traveling at more than one
half the velocity of light. When the electrons strike the surface of the
anode, they are slowed very quickly and lose their kinetic energy; the
kinetic energy is converted into either x-radiation or heat.
The electrons interact with individual atoms of the anode material, as shown
below. Two types of interactions produce radiation. An interaction with electron shells produces
characteristic x-ray photons; interactions with the atomic nucleus produce
Bremsstrahlung x-ray photons.
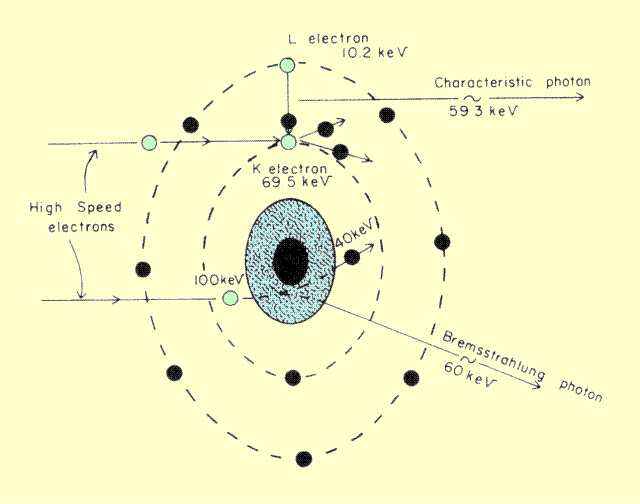
Electron-Atom Interactions That Produce X-Ray Photon | |
|
| |
Binding Energy |
|
|
The electrons within an atom each have a
specific amount of binding energy that depends on the size (atomic number,
Z) of the atom and the shell in which the electron is located. As
described in a previous chapter the binding energy is the energy that
would be required to remove the electron from the atom. It is
actually an energy deficit rather than an amount of available energy.
The binding energy of electrons within an atom plays a major role in the production of characteristic x-radiation as described later. | |
Production Process |
|
|
The interaction that produces the most photons is the Bremsstrahlung
process. Bremsstrahlung is a German word for "braking radiation" and is a
good description of the process. Electrons that penetrate the anode
material and pass close to a nucleus are deflected and slowed down by
the attractive force from the nucleus. The energy lost by the electron
during this encounter appears in the form of an x-ray photon. All
electrons do not produce photons of the same energy
| |
|
| |
Spectrum |
|
|
Only a few photons that have energies close to that of the electrons
are produced; most have lower energies. Although the reason for this is
complex, a simplified model of the
Bremsstrahlung interaction is shown below. First, assume that
there is a space, or field, surrounding the nucleus in which electrons
experience the "braking" force. This field can be divided into zones, as
illustrated. This gives the nuclear field the appearance of a target
with the actual nucleus located in the center. An electron striking
anywhere within the target experiences some braking action and produces
an x-ray photon. Those electrons striking nearest the center are
subjected to the greatest force and, therefore, lose the most energy and
produce the highest energy photons. The electrons hitting in the outer
zones experience weaker interactions and produce lower energy photons.
Although the zones have essentially the same width, they have different
areas. The area of a given zone depends on its distance from the
nucleus. Since the number of electrons hitting a given zone depends on
the total area within the zone, it is obvious that the outer zones
capture more electrons and create more photons. From this model, an
x-ray energy spectrum, such as the one shown
below, could be predicted.
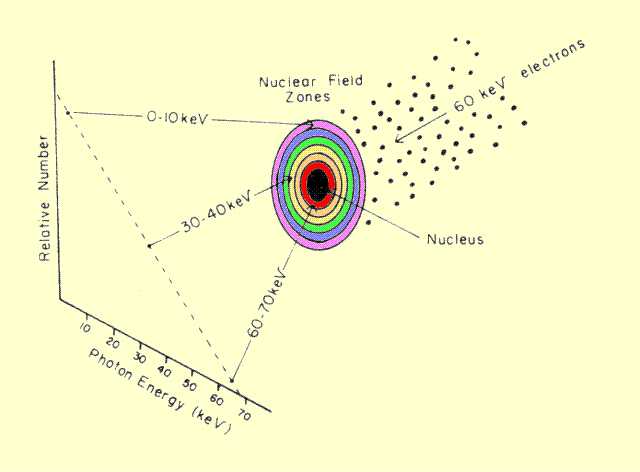
A Model for Bremsstrahlung Production and the Associated Photon Energy Spectrum The basic Bremsstrahlung spectrum has a maximum photon energy that corresponds to the energy of the incident electrons. This is 70 keV for the example shown. Below this point, the number of photons produced increases as photon energy decreases. The spectrum of x-rays emerging from the tube generally looks quite different from the one shown here because of selective absorption within the filter.
A significant number of the lower-energy photons are absorbed or
filtered out as they attempt to pass through the anode surface, x-ray
tube window, or added filter material. X-ray beam filtration is
discussed more extensively in
a later chapter. The amount of filtration is generally dependent
on the composition and thickness of material through which the x-ray
beam passes and is generally what determines the shape of the low-energy
end of the spectrum curve.
| |
|
| |
Effect of KV |
|
|
The high-energy end of the spectrum is determined by the KV
(kilovoltage) applied to the x-ray tube. This is because the
KV establishes the energy of the electrons as they reach the
anode, and no x-ray photon can be created with an energy greater than
that of the electrons. The maximum photon energy, therefore, in
keV is numerically equal to the maximum applied potential in kV
(kilovolts). In some x-ray equipment, the voltage applied to the tube
might vary during the exposure
because of the cycle nature of the AC (alternating current)
electrical
system. . The maximum photon energy is determined by the maximum,
or peak, voltage during the
voltage cycle. This value is generally referred to as the kilovolt
peak
(KVP) and is one of the adjustable factors of x-ray equipment.
In addition to establishing the maximum x-ray photon energy, the KVP
has a major role in determining the quantity of radiation produced for a
given number of electrons, such as 1 mAs, striking the anode. Since the
general efficiency of x-ray production by the Bremsstrahlung process is
increased by increasing the energy of the bombarding electrons, and the
electronic energy is determined by the
KVP, it follows that the
KVP affects x-ray production efficiency.
Changing the KVP
will generally alter the Bremsstrahlung spectrum, as shown
below. The total area under the spectrum curve represents the
number of photons
or quantity of radiation produced. If no filtration is present
where the spectrum is essentially a triangle, the amount of radiation
produced is approximately proportional to the KV squared. With the
presence of filtration, however, increasing the KV also increases the
relative penetration of the photons, and a smaller percentage is
filtered out. This results in an even greater increase in radiation
output with KVP.
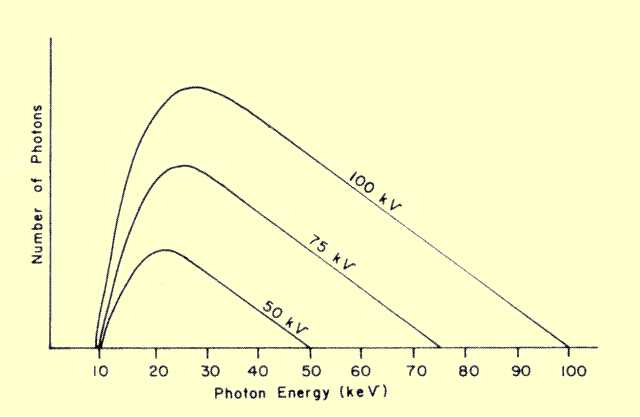 Comparison of Photon Energy Spectra Produced as Different KVP Values | |
|
| |
Production |
|
|
The type of interaction that produces characteristic radiation,
also illustrated
above (in the "Kinetic" paragraph), involves a collision between
the high-speed electrons and the orbital electrons in the atom. The
interaction can occur only if the incoming electron has a kinetic energy
greater than the
binding energy of the electron within the atom. When this
condition exists, and the collision occurs, the electron is dislodged
from the atom. When the orbital electron is removed, it leaves a vacancy
that is filled by an electron from a higher energy level. As the
filling electron moves down to fill the vacancy, it gives up energy
emitted in the form of an x-ray photon. This is known as characteristic
radiation because the energy of the photon is characteristic of the
chemical element that serves as the anode material. In the example
shown, the electron dislodges a tungsten K-shell electron, which has a
binding energy of 69.5 keV. The vacancy is filled by an electron from
the L shell, which has a binding energy of 10.2 keV. The characteristic
x-ray photon, therefore, has an energy equal to the energy difference
between these two levels, or 59.3 keV.
Actually, a given anode material gives rise to several characteristic
x-ray energies. This is because electrons at different energy levels (K,
L, etc.) can be dislodged by the bombarding electrons, and the
vacancies can be filled from different energy levels. The electronic
energy levels in tungsten are shown
below, along with some of the energy changes that give rise to
characteristic photons. Although filling L-shell vacancies generates
photons, their energies are too low for use in diagnostic imaging. Each
characteristic energy is given a designation, which indicates the shell
in which the vacancy occurred, with a subscript, which shows the origin
of the filling electron. A subscript alpha (a) denotes filling with an
L-shell electron, and beta ((3) indicates filling from either the M or N
shell
| |
|
| |
Tungsten Spectrum |
|
|
The spectrum of the significant characteristic radiation from tungsten is shown
below. Characteristic radiation produces a line spectrum with several discrete energies, whereas Bremsstrahlung produces a
continuous spectrum of photon energies over a specific
range. The number of photons created at each characteristic energy is
different because the probability for filling a K-shell vacancy is
different from shell to shell.
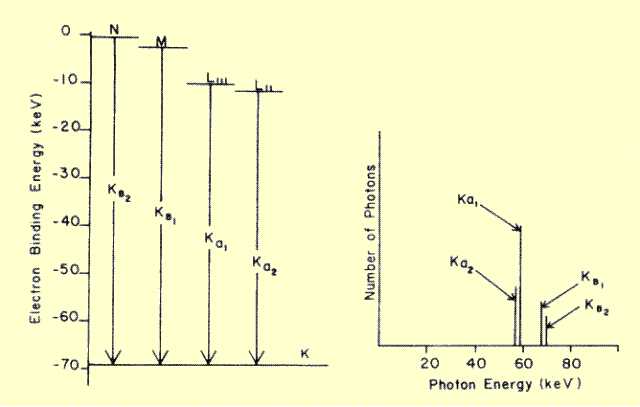 Electron Energy Levels in Tungsten and the Associated Characteristic X-Ray Spectrum | |
|
| |
Molybdenum Spectrum |
|
|
Molybdenum
anode tubes used for mammography produce two rather intense
characteristic x-ray energies: K-alpha radiation, at 17.9 keV, and
K-beta, at 19.5 keV.
as shown below.
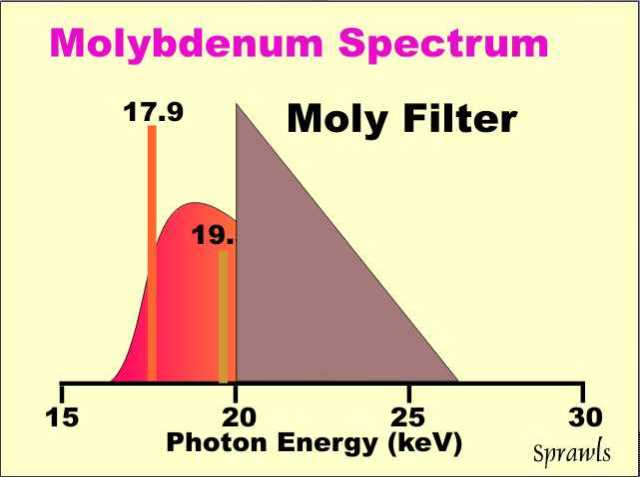
The optimum
spectrum to produce the best balance between contrast sensitivity and
radiation dose for an average size breast is one with most of the
radiation with photon energies below about 20 keV. However, there is
considerable Bremsstrahlung above this energy. In the typical mammography
equipment a molybdenum filter is used to remove that undesirable part of
the spectrum. This is an application of a filter that works on the
"K edge" principle. It absorbs radiation that is above the K-edge
energy that corresponds to the binding energy of the electrons in the K
shell of the molybdenum atom.
| |
Rhodium Spectrum |
|
|
Rhodium has an atomic number (Z) of 45 compared to a Z of
42 for molybdenum. Therefore the characteristic x-radiation produced with
a rhodium anode will have energies that are slightly higher than produced
with molybdenum and are more penetrating. This is of value for
imaging dense breast.
Anodes that have dual surface areas, molybdenum and rhodium, make it possible for the operator to select a spectrum that is more optimized for different breast sizes and densities. | |
KV Effect on Spectrum |
|
|
The KV value also strongly influences the production of characteristic
radiation. No characteristic radiation will be produced if the
KV is less (numerically) than the binding energy of the K-shell
electrons. When the
KV is increased above this threshold level, the quantity of
characteristic radiation is generally proportional to the difference
between the operating
KV and the threshold KV.
The x-ray beam that emerges from a tube has a spectrum of
photon energies determined by several factors. A typical spectrum is
shown
below and is made up of photons from both Bremsstrahlung and
characteristic interactions.
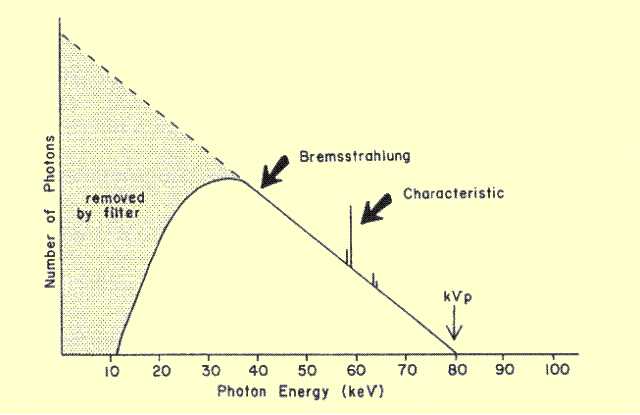 Typical Photon Energy Spectrum from a Machine Operating at KV = 80 The relative composition of an x-ray spectrum with respect to Bremsstrahlung and characteristic radiation depends on the anode material, KV, and filtration. In a tungsten anode tube, no characteristic radiation is produced when the KV is less than 69.5. At some higher KV values generally used in diagnostic examinations, the characteristic radiation might contribute as much as 25% of the total radiation. In molybdenum target tubes operated under certain conditions of KV and filtration, the characteristic radiation can be a major part of the total output. | |
Concept |
|
|
Only a small fraction of the energy delivered to the anode by the
electrons is converted into x-radiation; most is absorbed
by the anode and converted into heat. The efficiency of x-ray
production is defined as the total x-ray energy expressed as a fraction
of the total electrical energy imparted to the anode. The two factors
that determine production efficiency are the voltage applied to the
tube, KV, and the atomic number of the anode, Z. An approximate
relationship is
Efficiency = KV x Z x 10-6.
| |
KV Effect |
|
|
The relationship between x-ray production efficiency and KV
has a specific
effect on the practical use of x-ray equipment. As we will see in
a later chapter, x-ray tubes have a definite limit on the amount
of electrical energy they can dissipate because of the heat produced.
This, in principle, places a limit on the amount of x-radiation that can
be produced by an x-ray tube. By increasing KV, however, the quantity
of radiation produced per unit of heat is significantly increased.
| |
Anode Material |
|
|
The relationship of x-ray production efficiency to anode material is only
of academic interest because most tubes use tungsten. The exception is
molybdenum and rhodium used in mammography. The x-ray production
efficiency of these tubes is significantly less than that of tungsten
anode tubes because of their lower atomic numbers.
| |
Definition and Concept |
|
|
The x-ray efficacy of
the x-ray tube is defined as the amount of exposure, in milliroentgens,
delivered to a point in the center of the useful x-ray beam at a
distance of 1 m from the focal spot for 1 mAs of electrons passing
through the tube.
The efficacy value expresses the ability of a tube to
convert electronic energy into x-ray exposure. Knowledge of the
efficacy
value for a given tube permits the determination of both patient
and image
receptor exposures by methods discussed in later chapters. Like
x-ray energy output, the efficacy of a tube depends on a number of
factors including
KV, voltage waveform, anode material, filtration, tube age, and
anode surface damage.
The illustration below gives typical efficacy values for tungsten
anode tubes with normal filtration.
| |
KV Control |
|
|
KV
is very useful in controlling the radiation output of an x-ray tube.
The figure below shows a nonlinear relationship. It is normally
assumed that the radiation output is proportional to the square of the
KV. Doubling KV quadruples the exposure from the tube.
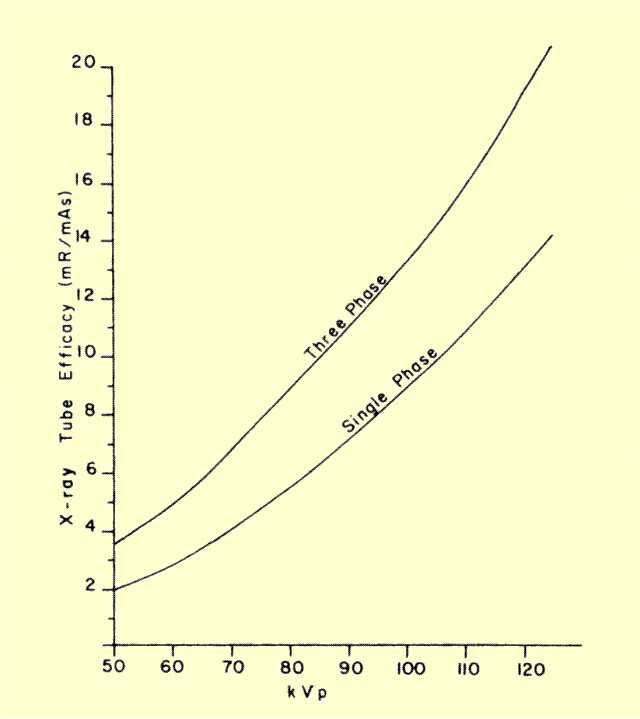 Typical X-Ray Tube Efficacy (Exposure Output) for Different KVP Values | |
Waveform |
|
|
Waveform describes the manner in which the KV changes with time during the x-ray production process
because of the cyclic nature of the electrical supply.; several different
KV waveforms are used. A general principle is that the waveform with the
least KV variation during the exposure is the most effective x-ray
producer. Most new x-ray equipment now use generators that produce
relatively constant KV throughout the exposure. Other waveforms are described in more
detail in another chapter.
| |
SUMMARY and MIND MAP |
|
The mindmap below provides a summary of the major concepts associated with x-ray production. | |
 | |


No comments:
Post a Comment