CONTRAST MEDIUM PREPARATIONS
There are three contrast medium preparations used in x-ray examinations ; Barium (Ba), Iodine (I) and Thorium (Th) but generally only Barium and Iodine preparation that still used in x-ray examination.
BARIUM PREPARATIONS
This is a suspension of powdered barium sulphate in a water. Barium sulphate is insoluble and chemically quite inert. Soluble salts of barium are very poisonous and only pharmaceutical quality barium sulphate should be used. Barium depends for its radiopacity on its electron density (reflected indirectly by its atomic number) which is much greater than the radio-opacity of soft-tissue and greater than the radio-opacity of bone. Barium sulfate, an insoluble white powder. This is mixed with water and some additional ingredients to make the contrast agent. As the barium sulfate doesn't dissolve, this type of contrast agent is an opaque white mixture. It is only used in the digestive tract; it is usually administered as an enema (for osepahogography, gaster, and intestinum tenue) or via rectal for colon.. After the examination, it leaves the body with the feces.
Should barium leak from the G.I. tract into tissues or into a body cavity eg. mediastinum or peritoneum, it can cause a fibrogranulomatous reaction. Spill into the bronchial tree is a manageable problem unless it is gross, when death may ensue; weak barium preparations have been used for bronchography. After oral administration, it may compact in the large bowel causing constipation and occasionally may precipitate obstruction if there is a predisposing pathology.
The ideal barium sulphate/water mixture has yet to be developed, but the following properties are of utmost importance.
a) Particle size. Ordinary barium sulphate particles are coarse, measuring several millimetres in size, but ultrafine milling of the crude barium sulphate results in 50 per cent of the particles having a size of between 5 Ecm and l5lCm. As rate of sedimentation is proportional to particle size, the smaller the barium sulphate particle the more stable the suspension.
(b) Non-ionic medium. The charge on the barium sulphate particle influences the rate of aggregation of the particles. Charged particles attract each other and thus form larger particles which sediment more readily. They tend to do this even more in the gastric contents and consequently sediment more readily in the stomach.
(c) pH of the solution. The pH of the barium sulphate solution should be around 5•3, as more acid solutions tend to become more so in the gastric contents and consequently precipitate more readily in the stomach.
(d) Palatability. Undoubtedly ultrafine milling reduces much of the chalky taste inherent in any barium sulphate/water mixture, but many commercial preparations contain a flavouring agent which further disguises the unpleasant taste. The barium sulphate/water mixture is usually 1/4 weight/volume, and has a viscosity of 15-20 cp, but thicker or thinner suspensions may be used. Many commercial preparations contain carboxymethyl cellulose (Raybar, Barosperse), which retains fluid and prevents precipitation of the barium suspension in the normal small bowel.
The development of the double contrast technique has stressed the need for adequate mucosal coating and much of the present manufacturing efforts are devoted to achieving this. An excess of mucus and undue collection of fluid in the stomach greatly inhibit adequate coating of the gastric mucosa, as does hypermotility of the stomach.
To achieve double contrast examination of the stomach, air or carbon dioxide gas must be introduced and there is no doubt that introduction of air or gas via a nasogastric tube is the best means of obtaining a controlled degree of gastric distension. However, the passage of a gastric tube is an unpleasant procedure and is not acceptable to all patients. Consequently most radiologists use effervescent tablets (sodium bicarbonate 35 mg, tartaric acid 35 mg, calcium carbonate 50 mg) to react with the gastric contents to produce carbon dioxide.
The amount of gas produced by these methods is variable and overdistension of the stomach in the double contrast technique associated with poor coating can be, from a diagnostic viewpoint, as disastrous as inadequate distension. Some commercial preparations contain carbon dioxide gas under pressure in the barium mixture, but usually the quantity of gas is not adequate to produce good double contrast meals. An anti-foaming agent may need to be added to some barium preparations to avoid the formation of bubbles.
Water soluble iodine-containing contrast media are of value when there is a suspected perforation or leakage of an anastomosis after operation. The low radio-opacity of the iodine compared with the barium, and the high osmolarity which results in dilution within the small bowel, make it of little value for routine use in investigation of the small bowel. Water soluble contrast media are contraindicated if there is any danger of aspiration into the lungs.
IODINE PREPARATIONS
Since their introduction in the 1950s, organic radiographic iodinated contrast medium (ICM) have been among the most commonly prescribed drugs in the history of modern medicine. The phenomenon of present-day radiologic imaging would be lacking without these agents. ICM generally have a good safety record. Adverse effects from the intravascular administration of ICM are generally mild and self-limited; reactions that occur from the extravascular use of ICM are rare. Nonetheless, severe or life-threatening reactions can occur wAll currently used ICM are chemical modifications of a 2,4,6-tri-iodinated benzene ring. They are classified on the basis of their physical and chemical characteristics, including their chemical structure, osmolality, iodine content, and ionization in solution. In clinical practice, categorization based on osmolality is widely used. ith either route of administration.
Types of Iodinated Contrast Medium
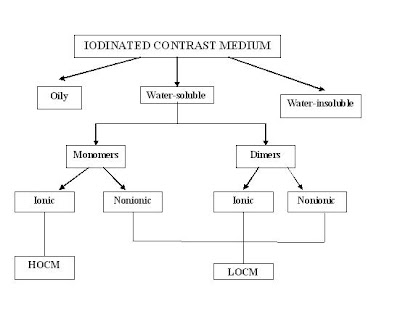
The more iodine, the more "dense" the x-ray effect. There are many different molecules. Some examples of organic iodine molecules are iohexol, iodixanol, ioversol. Iodine based contrast media are water soluble and as harmless as possible to the body. These contrast medium are sold as clear colorless water solutions, the concentration is usually expressed as mg I/ml. Modern iodinated contrast medium can be used almost anywhere in the body. Most often they are used intravenously, but for various purposes they can also be used intraarterially, intrathecally (the spine) and intraabdominally - just about any body cavity or potential space. The contras medium both ionic and non-ionic consist of monomer (1 benzoate acid ring) and dimmer ( 2 benzoate acid ring).
High-osmolality contrast media
Ionic monomers
High-osmolality contrast media consist of a tri-iodinated benzene ring with 2 organic side chains and a carboxyl group. The iodinated anion, diatrizoate or iothalamate, is conjugated with a cation, sodium or meglumine; the result is an ionic monomer. The ionization at the carboxyl-cation bond makes the agent water soluble. Thus, for every 3 iodine atoms, 2 particles are present in solution (ie, a ratio of 3:2).
The osmolality in solution ranges from 600 to 2100 mOsm/kg, versus 290 mOsm/kg for human plasma. The osmolality is related to some of the adverse events of these contrast media.Ionic monomers are subclassified by the percentage weight of the contrast agent molecule in solution

Low-osmolality contrast media
There are 3 types of low-osmolality ICM:, (1) ionic dimers, (2) nonionic monomers and (3) nonionic dimers.
Ionic dimers
Ionic dimers are formed by joining 2 ionic monomers and eliminating 1 carboxyl group. These agents contain 6 iodine atoms for every 2 particles in solution (ie, a ratio of 6:2). The only commercially available ionic dimer is ioxaglate. Ioxaglate has a concentration of 59%, or 320 mg I/mL, and an osmolality of 600 mOsm/kg. Because of its high viscosity, ioxaglate is not manufactured at higher concentrations. Ioxaglate is used primarily for peripheral arteriography.
Nonionic monomers
In nonionic monomers, the tri-iodinated benzene ring is made water soluble by the addition of hydrophilic hydroxyl groups to organic side chains that are placed at the 1, 3, and 5 positions. Lacking a carboxyl group, nonionic monomers do not ionize in solution. Thus, for every 3 iodine atoms, only 1 particle is present in solution (ie, a ratio of 3:1). Therefore, at a given iodine concentration, nonionic monomers have approximately one half the osmolality of ionic monomers in solution. At normally used concentrations, 25-76%, nonionic monomers have 290-860 mOsm/kg.
Nonionic monomers are subclassified according to the number of milligrams of iodine in 1 mL of solution (eg, 240, 300, or 370 mg I/mL).
The larger side chains increase the viscosity of nonionic monomers compared with ionic monomers. The increased viscosity makes nonionic monomers harder to inject, but it does not appear to be related to the frequency of adverse events.
Common nonionic monomers are iohexol, iopamidol, ioversol, and iopromide.
The nonionic monomers are the contrast agents of choice. In addition to their nonionic nature and lower osmolalities, they are potentially less chemotoxic than the ionic monomers.

Nonionic dimers
Nonionic dimers consist of 2 joined nonionic monomers. These substances contain 6 iodine atoms for every 1 particle in solution (ie, ratio of 6:1). For a given iodine concentration, the nonionic dimers have the lowest osmolality of all the contrast agents. At approximately 60% concentration by weight, these agents are iso-osmolar with plasma. They are also highly viscous and, thus, have limited clinical usefulness
An older type of contrast medium, Thorotrast was based on thorium dioxide, but this was abandoned since it turned out to be carcinogenic.
Properties of Iodine Contrast Medium
Osmolality, viscosity, and iodine concentration are three physico-chemical properties that are inter-related with each other, and are also influenced vy the structure and size of the iodine-binding molecule. The expression of each property can vary greatly among contras medium
During the last decade, innovations in the field of X-ray contras medium have focused on manipulation of these properties. However, due to their relatedness, a change in one property may cause a change in another one, at times unfavourably so. For example, effort to decrease osmolality have led to iso-osmolar product however, it has been at the cost of an unwanted higher level of viscosity.
Osmolality is a count of the number of particles in a fluid sample. The unit for counting is the mole which is equal to 6.02 x 1023 particles (Avogadro's Number). Molarity is the number of particles of a particular substance in a volume of fluid (eg mmol/L) and molality is the number of particles disolved in a mass weight of fluid (mmol/kg). Osmolality is a count of the total number of osmotically active particles in a solution and is equal to the sum of the molalities of all the solutes present in that solution. For most biological systems the molarity and the molality of a solution are nearly exactly equal because the density of water is 1 kg/L. There is a slight difference between molality and molarity in plasma because of the non-aqueous components present such as proteins and lipids which make up about 6% of the total volume. Thus serum is only 94% water and the molality of a substance in serum is about 6% higher than its molarity.
Osmolality can be calculated by the following formulation :

Many of the side effects are due to the hyperosmolar solution being injected. i.e. they deliver more iodine atoms per molecule.
Side-effects of Iodine contrast medium (ICM)
The use of Iodine contrast medium (ICM) may cause untoward side ffects and manifestations of anaphylaxis. The symptoms include nousea, vomiting, widespread erythema, generalized heat sensation, headache, coryza or laryngeal edema, fever, sweating, asthenia, dizziness, pallor, dyspnoea and moderate hypotension. More severe reaction involving the cardiovasluar system such as peripheral vasodilation with pronounced hypotension, tachycardia, dyspnoea, agitation, cyanosis and loss of consciousness, may require emergency treatment. For these reason the use of contrast medium must be limited to cases for which the diagnostic procedure is definitely indicated
Side effects in association with the intravascular use of iodinated contrast medium are ussually of a mild to moderate and temporary nature, and are less frequent with non-ionic than with ionic preparations..
Adverse reactions to ICM are classified as idiosyncratic and nonidiosyncratic.The pathogenesis of such adverse reactions probably involves direct cellular effects; enzyme induction; and activation of the complement, fibrinolytic, kinin, and other systems.
Idiosyncratic reactions
Idiosyncratic reactions typically begin within 20 minutes of the ICM injection, independent of the dose that is administered. A severe idiosyncratic reaction can occur after an injection of less than 1 mL of a contrast agent.
Although reactions to ICM have the same manifestations as anaphylactic reactions, these are not true hypersensitivity reactions. Immunoglobulin E (IgE) antibodies are not involved. In addition, previous sensitization is not required, nor do these reactions consistently recur in a given patient. For these reasons, idiosyncratic reactions to ICM are called anaphylactic reactions.
Anaphylactoid reactions
Anaphylactoid reactions occur rarely (Karnegis and Heinz, 1979; Lasser et al, 1987; Greenberger and Patterson, 1988), but can occur in response to injected as well as oral and rectal contrast and even retrograde pyelography. They are similar in presentation to anaphylactic reactions, but are not caused by an IgE-mediated immune response. Patients with a history of contrast reactions, however, are at increased risk of anaphylactoid reactions (Greenberger and Patterson, 1988; Lang et al, 1993). Pretreatment with corticosteroids has been shown to decrease the incidence of adverse reactions (Lasser et al, 1988; Greenberger et al, 1985; Wittbrodt and Spinler, 1994). The symptoms of anaphylactic reaction can be classified as mild, moderate, and severe.
Mild symptoms
Mild symptoms include the following: scattered urticaria, which is the most commonly reported adverse reaction; pruritus; rhinorrhea; nausea, brief retching, and/or vomiting; diaphoresis; coughing; and dizziness. Patients with mild symptoms should be observed for the progression or evolution of a more severe reaction, which requires treatment.
Moderate symptoms
Moderate symptoms include the following: persistent vomiting; diffuse urticaria; headache; facial edema; laryngeal edema; mild bronchospasm or dyspnea; palpitations, tachycardia, or bradycardia; hypertension; and abdominal cramps.
Severe symptoms
Severe symptoms include the following: life-threatening arrhythmias (ie, ventricular tachycardia), hypotension, overt bronchospasm, laryngeal edema, pulmonary edema, seizures, syncope, and death.
Anaphylactoid reactions range from urticaria and itching, to bronchospasm and facial and laryngeal edema. For simple cases of urticaria and itching, Benadryl (diphenhydramine) oral or IV is appropriate. For more severe reactions, including bronchospasm and facial or neck edema, albuterol inhaler, or subcutaneous or IV epinephrine, plus diphenhydramine may be needed. If respiration is compromised, an airway must be established prior to medical management.
Nonidiosyncratic reactions
Nonidiosyncratic reactions include the following: bradycardia, hypotension, and vasovagal reactions; neuropathy; cardiovascular reactions; extravasation; and delayed reactions. Other nonidiosyncratic reactions include sensations of warmth, a metallic taste in the mouth, and nausea and vomiting.
Bradycardia, hypotension, and vasovagal reactions
By inducing heightened systemic parasympathetic activity, ICM can precipitate bradycardia (eg, decreased discharge rate of the sinoatrial node, delayed atrioventricular nodal conduction) and peripheral vasodilatation. The end result is systemic hypotension with bradycardia. This may be accompanied by other autonomic manifestations, including nausea, vomiting, diaphoresis, sphincter dysfunction, and mental status changes. Untreated, these effects can lead to cardiovascular collapse and death. Some vasovagal reactions may be a result of coexisting circumstances such as emotion, apprehension, pain, and abdominal compression, rather than ICM administration.
Cardiovascular reactions
ICM can cause hypotension and bradycardia. Vasovagal reactions, a direct negative inotropic effect on the myocardium, and peripheral vasodilatation probably contribute to these effects. The latter 2 effects may represent the actions of cardioactive and vasoactive substances that are released after the anaphylactic reaction to the ICM. This effect is generally self-limiting, but it can also be an indicator of a more severe, evolving reaction.
ICM can lower the ventricular arrhythmia threshold and precipitate cardiac arrhythmias and cardiac arrest. Fluid shifts due to an infusion of hyperosmolar intravascular fluid can produce an intravascular hypervolemic state, systemic hypertension, and pulmonary edema. Also, ICM can precipitate angina.
The similarity of the cardiovascular and anaphylactic reactions to ICM can create confusion in identifying the true nature of the type and severity of an adverse reaction; this confusion can lead to the overtreatment or undertreatment of symptoms.
Other nonidiosyncratic reactions include syncope; seizures; and the aggravation of underlying diseases, including pheochromocytomas, sickle cell anemia, hyperthyroidism, and myasthenia gravis.
Extravasation
Extravasation of ICM into soft tissues during an injection can lead to tissue damage as a result of direct toxicity of the contrast agent or pressure effects, such as compartment syndrome.
Delayed reactions
Delayed reactions become apparent at least 30 minutes after but within 7 days of the ICM injection. These reactions are identified in as many as 14-30% of patients after the injection of ionic monomers and in 8-10% of patients after the injection of nonionic monomers.
Common delayed reactions include the development of flulike symptoms, such as the following: fatigue, weakness, upper respiratory tract congestion, fevers, chills, nausea, vomiting, diarrhea, abdominal pain, pain in the injected extremity, rash, dizziness, and headache.
Less frequently reported manifestations are pruritus, parotitis, polyarthropathy, constipation, and depression.
These signs and symptoms almost always resolve spontaneously; usually, little or no treatment is required. Some delayed reactions may be coincidental.
Nephropathy
Contrast-induced nephropathy is defined as either a greater than 25% increase of serum creatinine or an absolute increase in serum creatinine of 0.5 mg/dL. Three factors have been associated with an increased risk of contrast-induced nephropathy: preexisting renal insufficiency (such as Creatinine clearance < 60 mL/min [1.00 mL/s] - calculator online calculator), preexisting diabetes, and reduced intravascular volume (McCullough, 1997; Scanlon et al, 1999).
The osmolality of the contrast mdium is believed to be of great importance in contrast-induced nephropathy. Ideally, the contrast agent should be isoosmolar to blood. Modern iodinated contrast medium are non-ionic, the older ionic types caused more adverse effects and are not used much anymore.
To minimize the risk for contrast-induced nephropathy, various actions can be taken if the patient has predisposing conditions. These have been reviewed in a meta-analysis.
1. The dose of contrast medium should be as low as possible, while still being able to perform the necessary examination.
2. Non-ionic contrast medium
3. Iso-osmolar, nonionic contrast medium. One randomized controlled trial found that an iso-osmolar, nonionic agent was superior to a non-ionic agent contrast media.
4. IV fluid hydration with saline. There is debate as to the most effective means of IV fluid hydration. One method is 1 mg/kg per hour for 6-12 hours before and after the the contrast.
5. IV fluid hydration with saline plus sodium bicarbonate. As an alternative to IV hydration with plain saline, administration of sodium bicarbonate 3 mL/kg per hour for 1 hour before , followed by 1 mL/kg per hour for 6 hours after contrast was found superior to plain saline on one randomized controlled trial. This was subsequently corroborated by a multi-center randomized controlled trial, which also demonstrated that IV hydration with sodium bicarbonate was superior to 0.9% normal saline. The renoprotective effects of bicarbonate are thought to be due to urinary alkalinization, which creates an environment less amenable to the formation of harmful free radicals.
6. N-acetylcysteine (NAC). NAC, 600 mg orally twice a day, on the day before and of the procedure if creatinine clearnace is estimated to be less than 60 mL/min [1.00 mL/s]). A randomized controlled trial found higher doses of NAC (1200-mg IV bolus and 1200 mg orally twice daily for 2 days) benefited (relative risk reduction of 74%) patients receiving coronary angioplasty with higher volumes of contrast . Some recent studies suggest that N-acetylcysteine protects the kidney from the toxic effects of the contrast agent (Gleeson & Bulugahapitiya 2004). This effect is, in any case, not overwhelming. Some researchers (e.g. Hoffmann et al 2004) even claim that the effect is due to interference with the creatinine laboratory test itself. This is supported by a lack of correlation between creatinine levels and cystatin C levels.
Other pharmacological agents, such as furosemide, mannitol, theophylline, aminophylline, dopamine, and atrial natriuretic peptide have been tried, but have either not had beneficial effects, or had detrimental effects (Solomon et al, 1994; Abizaid et al, 1999).

No comments:
Post a Comment